A previous edition of EGG-NEWS confirmed widespread salmonellosis attributed to cantaloupes imported into both U.S. and Canada from Mexico. According to FDA import alert #22-1 dated December 20th, cantaloupes will be detained without physical examination unless supplied by a company appearing on an approved Green List.

Mexico has history of supplying cantaloupes with Salmonella contamination including multi- state outbreaks associated with S. Poona and S. Anatum.
Cantaloupes can be contaminated with Salmonella by contact with irrigation water containing either sewage or runoff from CAFOs, using contaminated water in cleaning and cooling, from insect and rodent pests, deficiencies in personal hygiene and cleaning of plants and equipment.
Field investigations by FDA in Mexico disclosed deviations from acceptable hygienic practices in cultivation, processing and packaging. In addition, cantaloupes from different areas are comingled complicating the process of traceback in the event of a foodborne outbreak.
|
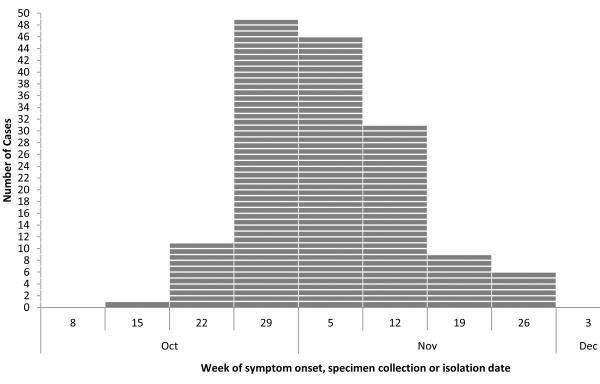
Epicurve of cases of salmonellosis in Canada attributed to cantaloupes from Mexico through November 2023
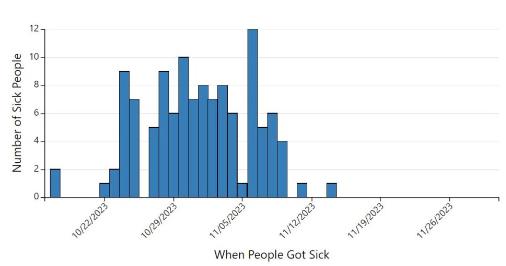
Epicurve Salmonella cases in the U.S. attributed to cantaloupes through November 2023
|
In 2005 the FDA entered into a memorandum of understanding with SENASICA, the Federal agency responsible for food safety in Mexico. The objective was to identify and authorize suppliers capable of delivering a non-contaminated product. Companies were evaluated according to their irrigation practices, packing and cooling, worker health and hygiene, sanitary facilities in field and packing houses and transportation.
Companies not currently approved under the Green List are required to demonstrate five shipments to be free of Salmonella and to comply with acceptable standards throughout the production chain.
Based on the incidence rate of salmonellosis in both the U.S. and Canada and the severity of infection that emerged in October 2023, the action by FDA, although belated, FDA action is justified by 302 confirmed cases in the U.S. in 42 states and 153 cases in Canada with a total of 11 fatalities through mid-December 2023.