 The National Institute of Allergy and Infectious Diseases of the National Institute of Health will commence a Phase-one trial to determine the efficacy of a broad-spectrum influenza vaccine designated BPL-1357. This candidate incorporates whole virus suspensions from four strains of chemically inactivated low-pathogenicity avian influenza virus. Preliminary studies in mice and ferrets demonstrated protection against potentially lethal doses of six influenza virus strains not included in the vaccine.
The National Institute of Allergy and Infectious Diseases of the National Institute of Health will commence a Phase-one trial to determine the efficacy of a broad-spectrum influenza vaccine designated BPL-1357. This candidate incorporates whole virus suspensions from four strains of chemically inactivated low-pathogenicity avian influenza virus. Preliminary studies in mice and ferrets demonstrated protection against potentially lethal doses of six influenza virus strains not included in the vaccine.
The evaluation will comprise a double-blind randomized trial with the following treatments:
- Group A participants will receive BPL-1357 by the intramuscular route with an intranasal saline placebo
- Group B will receive BPL-1357 by the intranasal route with an intramuscular placebo
- Controls will receive both intramuscular and intranasal placebo
The vaccination will be administered twice, separated by 28 days. The duration of the study will be seven months with assay of serum and nasal mucosal samples to monitor the immune response.
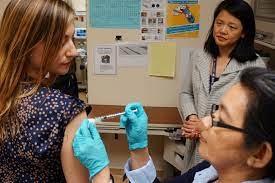 Dr. Matthew Memoli, principal investigator commented, “We are attempting to induce a comprehensive immune response that closely mimics immunity gained following a natural influenza infection.” He added, “This is very different than nearly all other vaccines for influenza or other respiratory viruses which focus on inducing immunity to a single viral antigen and often do not induce mucosal immunity.”
Dr. Matthew Memoli, principal investigator commented, “We are attempting to induce a comprehensive immune response that closely mimics immunity gained following a natural influenza infection.” He added, “This is very different than nearly all other vaccines for influenza or other respiratory viruses which focus on inducing immunity to a single viral antigen and often do not induce mucosal immunity.”
The results of this trial if positive may eventually be applicable to commercial poultry. If intranasal administration provides protection in humans, it is possible that the technology could be adapted to mass administration allowing for protection of large populations. This approach may be required as there is evidence that avian influenza strains including H5N1 may become endemic among migratory and resident wild birds and to continually expose poultry populations.