|
There has been no apparent progress in developing regulations relating to gene editing despite a memorandum of understanding between the USDA and the Department of Health and Human Services, the agency responsible for the FDA.
 The House Agriculture Committee was concerned over lack of progress during the third quarter of 2021 pointing to the structural restraints inherent in the division of responsibility between two agencies that demonstrate a disinclination or lack of ability to cooperate. The House Agriculture Committee was concerned over lack of progress during the third quarter of 2021 pointing to the structural restraints inherent in the division of responsibility between two agencies that demonstrate a disinclination or lack of ability to cooperate.
The signatories to the letter addressed to the USDA include the American Farm Bureau Federation, National Cattlemen's Beef Association, National Association of State Departments of Agriculture, National Turkey Federation, National Milk Producers’ Federation, and the American Soybean Association among others.
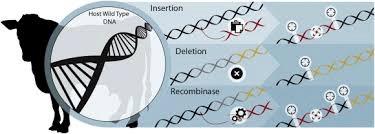
Delays inherent in the system of approval are exemplified by the decades required to finalize the application for Aquabounty genetically-engineered salmon. With the advent of CRISPR technology it is now possible to produce gene-deleted animals that show resistance to specific diseases or to climatic extremes contributing to productivity and sustainability.
The logjam with respect to approval of GM modification of livestock either by gene insertion or deletion reinforces the need for an independent food safety agency that will incorporate the current responsibilities for food safety exercised by the FDA and the oversight of inspection and regulations relating to red meat and poultry falling under the USDA-FSIS.
|