FDA Authorizes Second Booster Dose Of mRNA Vaccine To Protect Against COVID
|
04/06/2022 |
|
 On March 29th, the U.S. Food and Drug Administration authorized a second booster dose for recipients over the age of 50 and for those with an immunocompromised status. Essentially, the initial two-dose regimen was a primer to stimulate immunity followed by a suggested booster. This three-dose program should effectively be regarded as “fully vaccinated”. Due to waning immunity, the elderly and those who are immunosuppressed may benefit from a second booster using one of the two authorized mRNA vaccines”. On March 29th, the U.S. Food and Drug Administration authorized a second booster dose for recipients over the age of 50 and for those with an immunocompromised status. Essentially, the initial two-dose regimen was a primer to stimulate immunity followed by a suggested booster. This three-dose program should effectively be regarded as “fully vaccinated”. Due to waning immunity, the elderly and those who are immunosuppressed may benefit from a second booster using one of the two authorized mRNA vaccines”.
For the record, this commentator received the second booster (or fourth dose) vaccine on Friday, April 1st, resulting in mild pain at the site of injection and transitory fatigue for over half a day. This is a small price to pay for an assurance that, in the event of exposure to any of the current and the previous strains of SARS-COVID-19, symptoms will be mild and hospitalization can be regarded as an extremely unlikely outcome.
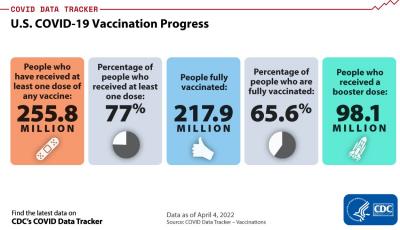
As at April 3rd, 562 million COVID vaccines, predominantly the two brands of mRNA products have been administered to the U.S. population. A total of 256 million (77.6 percent of the total population) have received one dose; 218 million, two doses regarded as “fully vaccinated” (66.1 percent) and 98 million have received a third ‘booster’ dose (29.8 percent) conferring immunity.
|
|
|

|
|
|