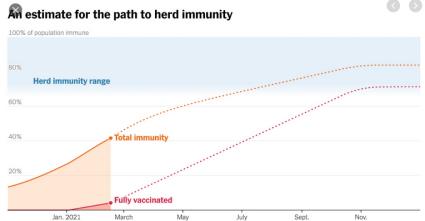
 The Emergency Use Authorization for COVID Vaccines to be administered to children aged from 12 to 16 is welcome news. This age cohort, if protected, will advance the level of herd immunity and facilitate return to summer camp and schools in the fall. This would be a further indication of a return to pre-COVID normality and expedite economic recovery.
The Emergency Use Authorization for COVID Vaccines to be administered to children aged from 12 to 16 is welcome news. This age cohort, if protected, will advance the level of herd immunity and facilitate return to summer camp and schools in the fall. This would be a further indication of a return to pre-COVID normality and expedite economic recovery.
Recent data suggests that the rate of COVID infection in teenagers is now exceeding that of seniors, 80 percent of whom have been vaccinated. Concurrently, hospitalizations for children due to COVID and its secondary effects are rising at a rate greater than patients over the age of 18. Pediatricians are concerned that variants may be  more pathogenic in children. Multisystem Inflammatory Syndrome is diagnosed more frequently in hospitals and surpassed 3,000 diagnosed cases by April 1st. Current COVID vaccines are effective against the B.1.1.7 variant that is more contagious than the original virus and by early April, was responsible for 60 percent of U.S. cases irrespective of age.
more pathogenic in children. Multisystem Inflammatory Syndrome is diagnosed more frequently in hospitals and surpassed 3,000 diagnosed cases by April 1st. Current COVID vaccines are effective against the B.1.1.7 variant that is more contagious than the original virus and by early April, was responsible for 60 percent of U.S. cases irrespective of age.
The CDC is concerned that children will be at risk in states with low vaccination rates including Mississippi, Louisiana, Alabama, Wyoming, and Idaho. With the coming of summer, after- school programs, socializing, and extracurricular activities is expected to increase the rate of transmission in non-vaccinated teens.