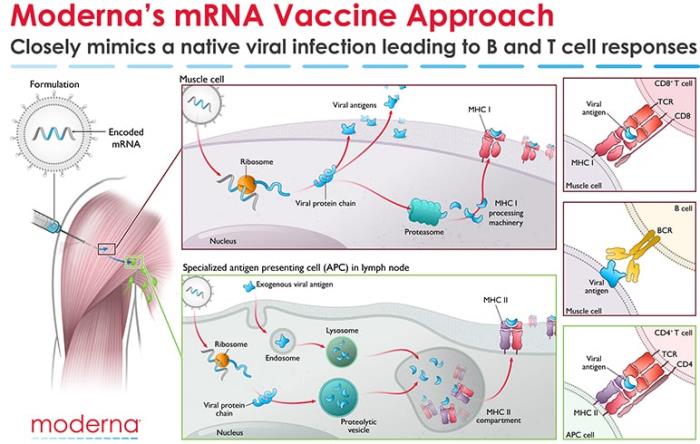
A publication in the New England Journal of Medicine* confirmed an overall 94.1 percent efficacy rate in protecting volunteers from COVID-19 in a Phase-3 trial. The data was evaluated by the FDA and CDC and the vaccine is now in distribution under experimental use authorization. The Moderna product, m-RNA-1273, is a lipid-nanoparticle-encapsulated mRNA vaccine expressing the perfusion-stabilized spike glycoprotein developed jointly by Moderna and the Vaccine Research Center at the National Institute of Allergy and Infectious Diseases (NIAID).
A publication in the New England Journal of Medicine* confirmed an overall 94.1 percent efficacy rate in protecting volunteers from COVID-19 in a Phase-3 trial. The data was evaluated by the FDA and CDC and the vaccine is now in distribution under experimental use authorization. The Moderna product, m-RNA-1273, is a lipid-nanoparticle-encapsulated mRNA vaccine expressing the perfusion-stabilized spike glycoprotein developed jointly by Moderna and the Vaccine Research Center at the National Institute of Allergy and Infectious Diseases (NIAID).
It is noted that despite media reports of rapid development of the vaccine under the WarpSpeed program, the basic research on developing m-RNA spike glycoprotein vaccines dates back to 2002 initiated during the SARS outbreak. Those questioning the speed of development should be assured that both the Pfizer and Moderna vaccines are based on sound concepts and research and were not the product of accelerated development in response to either epidemiologic or political pressure.
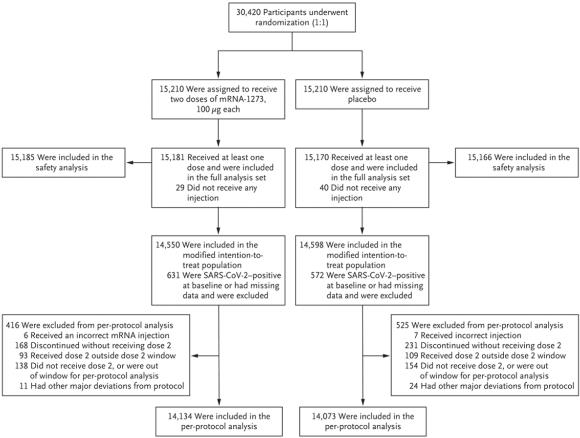
The Phase-3 trial of the Moderna vaccine was launched in July 2020 to assess safety and efficacy and involved 30,420 volunteers divided equally among placebo and vaccinated treatments. The placebo comprised 0.5 ml of saline. The vaccine was administered as 0.5 ml of an aqueous suspension of 100 micrograms of the encapsulated mRNA product. The Moderna vaccine requires storage at 35F to 46 F (refrigerator temperature) and is stable for eight hours at room temperature facilitating administration in remote locations.
Of the candidates enrolled, approximately 14,100 in each category completed the sequence of an initial vaccine and a booster at 28 days after the initial administration. Among the placebo group, there were 185 symptomatic cases of COVID-19 confirmed by RT-PCR assay. Within the vaccinates, 11 cases were confirmed, yielding an efficiency of 94.1 percent. Among the placebo group, there were 30 serious cases of COVID-19 and one fatality. There were no serious cases among the vaccinates. There was a wide range of demographic characteristics at baseline among the volunteers. Results were subdivided among ages with categories aged 18 to 65 and above 65; those at risk for severe consequences of COVID-19, male and female, and white versus communities of color. The lowest level of protection was 86.4 among those aged 65 years and older, irrespective of risk of contracting COVID-19. The highest level of protection was 97.5 percent among communities of color.
Since production of the Moderna vaccine is now scaling up in volume the challenge will be to administer the product at a rate equivalent to delivery to distribution centers.
*Baden, L.R. et al. Efficacy and Safety of the mRNA-1273 SARS-Co-V-2 Vaccine. New England Journal of Medicine. DOI:10.1056/NEJMoa2035389. December 30, 2020.