Therapeutics to be Evaluated in Phase Three Clinical Trials for COVID
|
12/23/2020 |
|
Despite the approval and release of two COVID-19 vaccines, there is an urgent need for effective therapy for the more than 200,000 new daily cases of the infection.
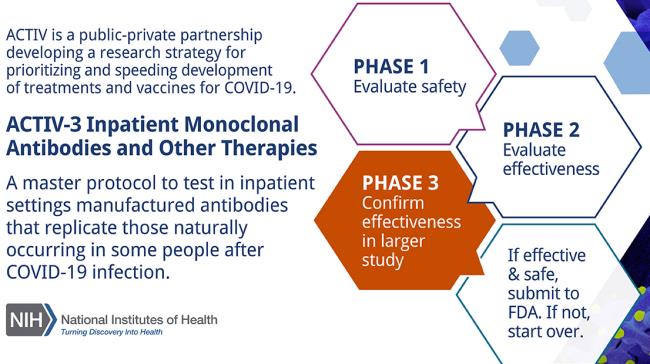
The ACTIVE-3 Program (Accelerating COVID-19 Therapeutic Interventions and Vaccine Partnership) is administered by the NIH. ACTIVE-3 previously tested a monoclonal antibody ly-coV555 developed by Eli Lilly and company. Investigators closed the study based on the “low likelihood that the intervention would be of clinical value to hospitalized patients.”
A new study will be initiated examining the therapeutic potential of VIR-7831, a monoclonal antibody developed jointly by GlaxoSmithKline of the U.K. and Vir Biotechnology in the U.S. The second monoclonal combination to be evaluated will be BrII-196 and BrII-198 manufactured by Brii Biosciences with locations in China and the U.S. Participants in the study will be randomized equally to receive either a placebo, VIR-7831 or the BrII combination. The initial trial will involve 450 hospitalized volunteers with mild to moderate COVID-19. If the antibody treatment appears safe and effective, an additional 700 volunteers will be enrolled.
|

NIH Campus Bethesda MD. |

|
|
|