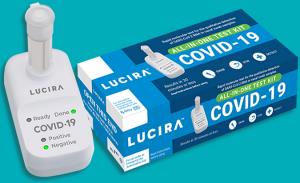
 The Food and Drug Administration has issued an emergency authorization for the Lucira COVID-19 all-in-one test kit. The system uses loop-mediated isothermal amplification with single tube detection with a simple reader that provides a qualitative (positive/negative) readout.
The Food and Drug Administration has issued an emergency authorization for the Lucira COVID-19 all-in-one test kit. The system uses loop-mediated isothermal amplification with single tube detection with a simple reader that provides a qualitative (positive/negative) readout.
 The manufacturers claim a 94 percent positive predictive value and 98 percent negative predictive value compared to standard PCR. Sensitivity and specificity data has yet to be released.
The manufacturers claim a 94 percent positive predictive value and 98 percent negative predictive value compared to standard PCR. Sensitivity and specificity data has yet to be released.
The system will be available for patients 14 years and older and will require a prescription. Projected cost is $50 per test that is acceptable for physician's office diagnosis but too expensive for regular self-home testing.
Lucira information can be obtained from the manufacturers' website <info@lucirahealth.com>.