 Collaboration between the Massachusetts Institute of Technology and manufacturer 3M has led to the development of a lateral flow immunoassay test kit to detect the presence of SARS-COV-2 antigen
Collaboration between the Massachusetts Institute of Technology and manufacturer 3M has led to the development of a lateral flow immunoassay test kit to detect the presence of SARS-COV-2 antigen 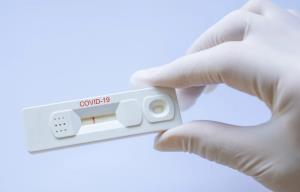 responsible for COVID-19. If the kit passes initial validation for sensitivity and specificity, additional Phase 2 evaluation will be required to achieve regulatory approval. John Banovetz Chief Technology Officer for 3M stated, “The holy grail of this would be something akin to a home pregnancy test kit.”
responsible for COVID-19. If the kit passes initial validation for sensitivity and specificity, additional Phase 2 evaluation will be required to achieve regulatory approval. John Banovetz Chief Technology Officer for 3M stated, “The holy grail of this would be something akin to a home pregnancy test kit.”
The system could be deployed at bedside, in the work place or at home. The advantage of speed is self-evident. Results could be obtained within an hour compared to the inordinate time required to submit a swab to a laboratory equipped with PCR assay capability.
From personal experience, it took three days to schedule a COVID-19 test in North Carolina and results are not anticipated for at least four days. The objective of testing is to determine that a  patient is in fact infected, with a reasonable level of sensitivity and specificity and to undertake quarantine with subsequent tracing of contacts. The seven-day delay between requiring a test based on symptoms or exposure and receiving the results is totally inadequate in the context of controlling the disease.
patient is in fact infected, with a reasonable level of sensitivity and specificity and to undertake quarantine with subsequent tracing of contacts. The seven-day delay between requiring a test based on symptoms or exposure and receiving the results is totally inadequate in the context of controlling the disease.
Presumptive positives identified using a lateral flow immunoassay test could be immediately isolated and the diagnosis confirmed by more sensitive PCR assay during quarantine.