A report in the peer-reviewed literature has been published on Phase 1 evaluation of a COVID-19 vaccine*. Dr. Lisa A. Jackson of Kaiser Permanente Washington Health Research Institute in Seattle in cooperation with scientist at Emory University in Atlanta provided data on the first trial monitored and supported by the National Institutes of Allergy and Infectious Diseases.
The trial initiated in March involved three groups of fifteen participants 18-55 years of age. One of three levels of vaccine was administered in two successive doses to determine safety and efficacy. The subjects developed antibodies with levels proportional to the quantum of mRNA received. The levels of antibody stimulated corresponded to convalescent sera obtained from patients recovering from COVID-19.
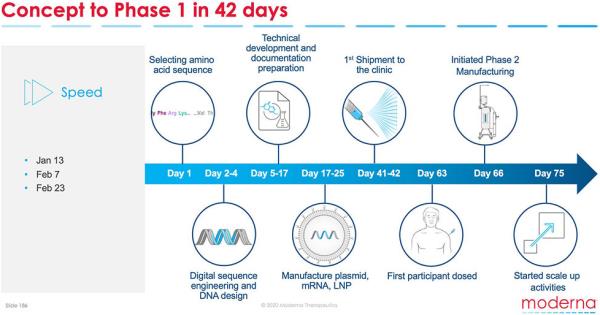
Development of Moderna COVID Vaccine
It was noteworthy that no serious adverse events were reported although only forty-five subjects were enrolled in the study. Half of the patients reported transitory mild symptoms including fatigue, headache, pain at the injection site especially following the second vaccination and in subjects receiving highest dose of the active ingredient.
A Phase-2 trial was initiated in May with a Phase-3 trial to commence in July.
It is evident that the World will only be able to resume pre-COVID economic activity with the assurance of a safe and effective vaccine. Various candidates are currently under evaluation but even if protective levels of antibody are stimulated and vaccines are innocuous it will take time to test, manufacture and distribute products. Administration and acceptance will be major challenges both in industrialized and developing nations. Unless we achieve 70 percent ‘herd immunity’ in populations by either immunization or exposure we will be faced with upsurges in incidence rates reflecting deficiencies in accepted preventive measures.
*Jackson, L. A. et al. A SARS-CoV-2 mRNA vaccine-Preliminary report New England Journal of Medicine. DOI;10.1056/NEJM oa2022483 (2020)