 It is thankfully apparent that at least the U.S. now has two COVID-19 candidate vaccines represented by the Pfizer-BioNTech and Moderna Therapeutics mRNA-based products. Both vaccines have proven effective and safe as demonstrated by extensive field trials reported in press releases by the two companies. Emergency Use Approval by the Food and Drug Administration is expected in late December following expedited but thorough scientific review.
It is thankfully apparent that at least the U.S. now has two COVID-19 candidate vaccines represented by the Pfizer-BioNTech and Moderna Therapeutics mRNA-based products. Both vaccines have proven effective and safe as demonstrated by extensive field trials reported in press releases by the two companies. Emergency Use Approval by the Food and Drug Administration is expected in late December following expedited but thorough scientific review.
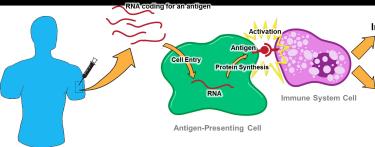
Both vaccines are based on mRNA coding for spike proteins of the virus that stimulate production of antibodies. Developed under the inappropriately named “Warp Speed” program and funded to a level of $10 billion both vaccines require and initial dose followed by a second ‘booster’ at intervals of 3 to 4 weeks depending on type and future field experience.
The Federal government funded the developers of vaccines to concurrently refine development of the antigenic mRNA and to establish a production capability so that vaccines would be available shortly after FDA approval. Approximately 50 million doses of the two vaccines will be ready for distribution during the first two months of 2021 with larger quantities thereafter.
 The Pfizer vaccine must be stored at -100F but remains potent for 10 days at -78F corresponding to the temperature of dry ice. The Moderna vaccine can be stored at -4F for extended periods but requires a temperature of 35 to 45F corresponding to a conventional refrigerator, for no longer than 30 days.
The Pfizer vaccine must be stored at -100F but remains potent for 10 days at -78F corresponding to the temperature of dry ice. The Moderna vaccine can be stored at -4F for extended periods but requires a temperature of 35 to 45F corresponding to a conventional refrigerator, for no longer than 30 days.
The Federal government has arranged for the military to coordinate the acquisition and distribution of vaccine and has stockpiled adequate quantities of vials, needles and other consumables to support a nationwide vaccination program. It is however evident that the commercial sector with experience in cold-chain logistics will have to be involved. Fedex and UPS will be the most likely carriers, medical supply companies such as McKesson will provide local storage and distribution. Pfizer has planned a $2 billion program to install low-temperature freezers in warehouses and has developed transport modules containing dry ice to support their product.
Pharmacies eligible under the Federal Pharmacy Partnerships Strategy for COVID-19 Vaccination will participate in administration to recipients. This may be impractical since the proposed COVID vaccines are more difficult to store and inject than an annual flu-shot. Even if half our population agree to receive the vaccine, with a two-dose program it will be necessary to administer 310 million doses in eight to ten months subject to the availability of vaccine. Given the stringent requirements for storage and handling of the Pfizer vaccine, it isdoubtful whether conventional pharmacies, whether as stores or located in supermarkets will be able to achieve the required rate of administration or to maintain viability of the product. (Given paperwork, an available pharmacist and no line of people waiting, my annual influenza vaccination in September took 30 minutes in a CVS pharmacy). Given the logistic complications involved and the need for rapid administration and to establish a reliable database, it will probably be necessary for recipients to attend vaccination clinics at hospitals, national guard armories or convention centers equipped to store and handle vaccine that must be administered at a high rate.
 The most pressing problem relates to whether citizens are willing to take the vaccine. Various surveys have shown a projected compliance rate of 20 to 50 percent. Unfortunately, the question of vaccination was politicized along with many aspects of the prevention and control of COVID including the collection, reporting and analysis of data. The selection of the term "Warp-Speed" for the development of the program was extremely inappropriate since it created an image of speed at the expense of science. Many prospective recipients are concerned over safety and to a lesser extent efficacy given the statements from Administration spokespersons and contradictory information from established public health specialists. Prospective recipients of vaccine have been confused by deliberate underplaying of the gravity of the Covid-19 epidemic in the U.S. We have been subject to official denial regarding the severity of the infection and the need for testing in the face of ascending incidence and fatality rates. The control of COVID-19 by non-medical modalities has not been supported by the Federal government and has been delegated (or abrogated) to states. Confidence in vaccines among our polarized citizenry has been adversely affected by denigrating and criticizing established and recognized professionals in the areas of epidemiology and those with experience in developing and administrating vaccines and the control of infectious diseases. Suspicion that agencies including the FDA, NIH and the CDC were subject to political pressure to approve ineffective therapeutics as a “quick-fix” expedient and downplaying the significance of COVID-19 have all generated a disinclination to be at the head of the line to receive a vaccination.
The most pressing problem relates to whether citizens are willing to take the vaccine. Various surveys have shown a projected compliance rate of 20 to 50 percent. Unfortunately, the question of vaccination was politicized along with many aspects of the prevention and control of COVID including the collection, reporting and analysis of data. The selection of the term "Warp-Speed" for the development of the program was extremely inappropriate since it created an image of speed at the expense of science. Many prospective recipients are concerned over safety and to a lesser extent efficacy given the statements from Administration spokespersons and contradictory information from established public health specialists. Prospective recipients of vaccine have been confused by deliberate underplaying of the gravity of the Covid-19 epidemic in the U.S. We have been subject to official denial regarding the severity of the infection and the need for testing in the face of ascending incidence and fatality rates. The control of COVID-19 by non-medical modalities has not been supported by the Federal government and has been delegated (or abrogated) to states. Confidence in vaccines among our polarized citizenry has been adversely affected by denigrating and criticizing established and recognized professionals in the areas of epidemiology and those with experience in developing and administrating vaccines and the control of infectious diseases. Suspicion that agencies including the FDA, NIH and the CDC were subject to political pressure to approve ineffective therapeutics as a “quick-fix” expedient and downplaying the significance of COVID-19 have all generated a disinclination to be at the head of the line to receive a vaccination.
Fortunately, the pharmaceutical industry and specifically the manufacturers of vaccines issued a joint statement in October confirming that they would only submit data and request FDA approval when they were confident that the vaccine was both safe and effective based on field trials. To this point there was concern that November 3rd was a critical date for approval based on political considerations. It will be the responsibility of the incoming Administration to restore confidence and to convince the public that the FDA will base approval on evaluation only of scientific data.
It is considered significant that the first action taken by president-elect Biden was to establish a COVID taskforce of twelve leading academics all with practical experience in areas of epidemiology and public health. Cooperation in the form of a joint transitional task force on planning and execution of a difficult vaccination program will be essential to successful protection against COVID-19 and saving lives.
It is an established fact that until COVID-19 is controlled, there cannot be a restoration of either a normal life or our economy. The alternative approach of ignoring COVID and making belief that it did not exist has resulted in over 10 million cases, 250,000 fatalities and billions of dollars in cost and hardship. We cannot look back but must make the best use of available vaccines, prioritized to our first line responders and those with predisposing conditions. Hopefully the initial vaccination program will be successful and that progressively more citizens will be immunized. The theory of herd immunity from natural exposure has been disproved and is now the preoccupation of political hacks, science deniers, charlatans and mountebanks. Ultimately, to control COVID-19 we will have to rely on a combination of common-sense precautions combined with effective vaccines. Until a high proportion of our population is immunized, we will be dependent on masking, social distancing and avoiding crowds. These precautions will be required as we attempt to "flatten the curve" for the third time.
We owe our scientists both in basic molecular biology and applied vaccinology our gratitude. We are indebted to first responders for the care of those who have contracted COVID and we extend our condolences to the bereaved among us as every one of the quarter million fatalities was once a living functioning person with a family, aspirations and future opportunities.