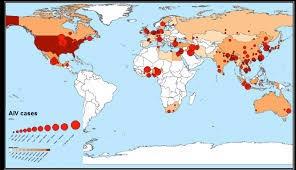
 The European Food Safety Agency recently updated statistics relating to outbreaks of Highly Pathogenic Avian Influenza (HPAI) in Europe. From October 2021 through September 2022, a total of 2,520 outbreaks have occurred in poultry with close to 4,000 detections in wild birds. To date, 50 million birds have been depopulated in an attempt to eradicate the infection. France recorded the highest number of outbreaks involving regions where pasture management of ducks, geese and chickens is followed. Despite mandatory housing requirements, HPAI has continued with a surge in cases reported following the fall southward migration of free-living birds. As with the U.S., there is evidence that domestic birds of diverse families are susceptible to HPAI strain H5N1, but it is unclear whether clinically unaffected birds can serve as disseminators of the virus and there is no reliable data on the duration of shedding. Again, in common with the U.S. and Canada in 2022, there was no period of quiescence in summer in the E.U. with sporadic outbreaks reported, consistent with spread of the virus by domestic, non-migratory birds.
The European Food Safety Agency recently updated statistics relating to outbreaks of Highly Pathogenic Avian Influenza (HPAI) in Europe. From October 2021 through September 2022, a total of 2,520 outbreaks have occurred in poultry with close to 4,000 detections in wild birds. To date, 50 million birds have been depopulated in an attempt to eradicate the infection. France recorded the highest number of outbreaks involving regions where pasture management of ducks, geese and chickens is followed. Despite mandatory housing requirements, HPAI has continued with a surge in cases reported following the fall southward migration of free-living birds. As with the U.S., there is evidence that domestic birds of diverse families are susceptible to HPAI strain H5N1, but it is unclear whether clinically unaffected birds can serve as disseminators of the virus and there is no reliable data on the duration of shedding. Again, in common with the U.S. and Canada in 2022, there was no period of quiescence in summer in the E.U. with sporadic outbreaks reported, consistent with spread of the virus by domestic, non-migratory birds.
The European Commission has requested the European Food Safety Agency to determine the availability and efficacy of vaccines against Highly Pathogenic Avian Influenza for poultry and to develop control strategies incorporating vaccination. The Wageningen Institute in Holland has initiated a trial to determine the level and duration of protection against clinical signs and shedding in vaccinated chickens and ducks using commercially available and experimental vaccines. Currently, vaccines are deployed in Egypt, and presumably other North African nations, and in Mexico.
 Vaccination against HPAI was the subject of an international conference convened by the International Alliance for Biological Standardization held in Paris, France on October 25th and 26th. This meeting considered a number of technical issues relating to vaccination but concentrated on trade and regulatory aspects that are advanced by opponents of vaccination.
Vaccination against HPAI was the subject of an international conference convened by the International Alliance for Biological Standardization held in Paris, France on October 25th and 26th. This meeting considered a number of technical issues relating to vaccination but concentrated on trade and regulatory aspects that are advanced by opponents of vaccination.
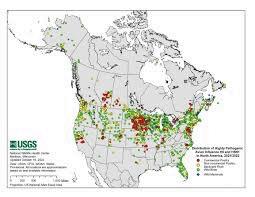
Given the depopulation of over 50 million commercial poultry in the U.S. since the beginning of the 2022 epornitic, confirmation of the presence of H5N1 HPAI virus in 45 states and continuing recovery from free-living birds with sporadic outbreaks in commercial poultry, the classification of HPAI in the U.S. as an “exotic” disease must be questioned. A further complication is the growing realization based on circumstantial and anecdotal reports that HPAI may be transmitted over short to intermediate distances by the aerogenous route as has been demonstrated with Newcastle disease. This mode of infection would under practical conditions negate currently applied structural and operational biosecurity, suggesting alternatives, including vaccination to protect poultry.
It is impossible to eradicate a highly infectious endemic disease over the short or even intermediate term and accordingly, the USDA is faced with the options of either running out of money and resources or ultimately birds to depopulate. The situation in Weld County, Colorado, with five outbreaks among four large egg-production complexes in six months is a case in point. Essentially the USDA-APHIS is approaching the current epornitic with the same mindset as the 1994 outbreaks in Pennsylvania and is following a whack-a-mole strategy reliant on Commodity Credit Corporation funding.

The time has come to seriously consider strategic application of vaccination as a component of control. If we regard avian influenza as the “Newcastle disease of the 2020s” the approach to control and prevention is self-evident. We have universal vaccination against this previously catastrophic infection, trade continues and the disease is not a restraint to production except in some Scandinavian nations where vaccination is disallowed.
Adoption of vaccination to develop immune populations eligible for trade is feasible based on improved diagnostic capability to certify flocks as free of infection. Advances in vaccination technology will hopefully provide protection through automated or mass administration, raising regional or national flock immunity above the outbreak threshold. If vaccination is to be adopted as a co-strategy with existing control measures it will be necessary to modify outdated restrictions that ignore the realities of extensive worldwide endemic HPAI infection.
 It is evident that opposition to vaccination incorporates irrelevant or spurious claims that are dated in the context of scientific advances and the reality of the ongoing World pandemic of H5N1 Avian Influenza. We need more field and molecular epidemiologic data on the mode of transmission of HPAI together with evidence that specific vaccines and their deployment will be effective in suppressing mortality and shedding. Above all we will require unanimity among segments of the U.S. poultry industry that effective vaccines will be commercially beneficial.
It is evident that opposition to vaccination incorporates irrelevant or spurious claims that are dated in the context of scientific advances and the reality of the ongoing World pandemic of H5N1 Avian Influenza. We need more field and molecular epidemiologic data on the mode of transmission of HPAI together with evidence that specific vaccines and their deployment will be effective in suppressing mortality and shedding. Above all we will require unanimity among segments of the U.S. poultry industry that effective vaccines will be commercially beneficial.